A hydrogen bond is an electrostatic attraction. It is formed when a hydrogen atom is covalently bound to a highly electronegative atom. This highly electronegative atom can be nitrogen, oxygen, fluorine etc. Two types of hydrogen bonds are there:
- Intermolecular Bonds– These bonds are formed between two atoms of different species, for e.g. bond formed in water molecules.
- Intramolecular bonds– These bonds are formed between two atoms of same species, for e.g. hydrogen bonds formed between 2nd and 5thcarbon in alpha helix structure of all biomolecules.
Three molecules having hydrogen bonds are:
- Water
- DNA
- Alpha-helical proteins (keratin)
Water: The structure of water molecules with hydrogen bonds looks like this.
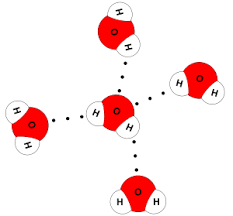

Here, oxygen is the highly electronegative species bonded to hydrogen. This is intermolecular hydrogen bonding because oxygen of one molecule is bonded to hydrogen of another molecule, and not the same molecule. Liquids like water which show hydrogen bonding are also known as “associated liquids.”
Hydrogen bonding affects physical and chemical properties of water.
Physical properties:
- The solid form of water is less dense than its liquid form.
- Water has high boiling point of 100 degree C.
- Water has high heat capacity
Chemical properties
- Water is a polar molecule. This is because of the hydrogen bonds between them. This polarity allows them to separate all ions into salts.
- Water makes strong bonds with polar substances like alcohols and acids; it dissolves these substances.
What will happen if hydrogen bonds were not there in the water?
- If hydrogen bond was not there, then there would be no ionic interaction between oxygen and hydrogen. As a result, water will no more remain liquid. In fact, it will become crystalline.
- One important point of consideration is that bond breakage is endothermic. If hydrogen bonds are there, then the formation of hydrogen bonds will require a lot of energy (endothermic process). So, water has a high boiling point because of the hydrogen bonds. If hydrogen bonds will not be there, then boiling point of water will reduce.
- Third, when sun’s heat falls on the sea, then water in sea never boils. This is because hydrogen bonding has conferred high boiling point to water. If there were no hydrogen bonds in water, then sea water would boil after receiving solar radiation. In such an environment, marine life would be impossible.
- Life on earth overall would also be impossible. This is so because humans would not be able to drink water in summers at all; as it would be too hot to drink. More than 70% of a human body which is water would be extremely hot.
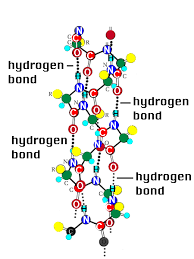
DNA
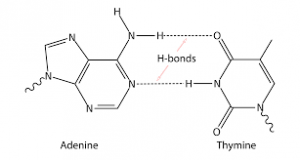
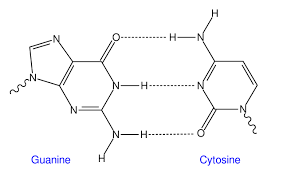
Deoxyribonucleic acid or DNA is the genetic material of all life forms on Earth. DNA is made up of nucleotides. Nucleotides contain deoxyribose sugar, phosphate, and base. There are four bases in DNA: adenine, guanine, cytosine, and thymine. Now, DNA is a double helical structure. Hydrogen bonding between bases joins two DNA strands together.
In forming the structure of DNA, adenine binds to thymine via two hydrogen bonds, while cytosine binds to guanine via three hydrogen bonds.
The hydrogen bonds of DNA will look somewhat like this:
Properties of DNA given by hydrogen bond:
- DNA absorbs UV light at 260nm. Double stranded DNA absorbs less UV light in comparison to single-stranded DNA. If hydrogen bond were not there, then DNA bases would have absorbed so much UV light, as to cause mutations and genetic drift every day.
- DNA has a different density in different regions. GC base pair has more density than AT base pair. This is because GC base pair has 3 hydrogen bonds in comparison to AT base pair which has two hydrogen bonds. These density differences account for different properties of DNA in different processes. For e.g. AT regions are present in start site of DNA replication process. It is easier to melt DNA having AT regions in comparison to GC regions.
- Density differences also help in understanding the repetitive sequences of DNA.
- The hydrogen bonding gives high melting temperature to DNA. Under normal conditions of heat, DNA cannot melt. It is important to note that if hydrogen bonding were not there, then DNA would melt in the extreme heat of summers when the temperature rises to 50 degrees Celcius.


Alpha-helical proteins (e.g. Keratin):
Keratin protein is present in human body. This protein is present in all epithelial cells. It protects the cells from damage or stress. It has alpha-helix structure. In alpha- helix, every N-H group of one amino acid donates its ‘H’ to C=O of 4thamino acid. So, there is a hydrogen bond between ‘H’ and ‘O’. Protein chain a long chain of amino acids. So, a long chain shortens into a small chain via the hydrogen bonds. The hydrogen bonds confer basic properties to alpha helical proteins:
- First, the protein length is shortened, as a result of which the protein is able to fit inside our body cells. So, in a way, we can say that hydrogen bond gives stability to the keratin protein.
- Second, hydrogen bonding gives high boiling point to these proteins. So, they can exist in high internal body temperature.
- All physical interactions of the protein are possible via this hydrogen bond. The hydrogen bond changes the shape of proteins from time to time. Transmembrane proteins are able to span the membranes with the help of this bonding.
- The structure of keratin protein can be summarized as follows: